a specific property of organic molecules that provides stability and reduces reactivity of compounds.
Origin: via Latin (aromatyk) from Greek (aromaticus)
Strangely enough, most aromatic compounds are rather foul smelling...
Today, I'm exhausted from writing midterms all week and thus have no interesting thoughts to share at the moment, so I will spend the first half of the blog helping you understand the chemical phenomenon known as Aromaticity, defined losely above.
Aromatic compounds are special in that they are much more stable than chemists would normally predict them to be. To understand aromaticity and its properties, it is essential to understand what defines an aromatic compound from a non-aromatic compound.
In organic chemistry, an aromatic compound is defined to be a molecule that possess both
a) a ring structure that is planar and has an unbroken/uninterrupted p-orbital loop both above and below the plane of the ring.
b) an odd number of pi-bonding electrons.
Ring structures are very common in biochemistry, and play a very important role in life: sugars, DNA, amino acids, and many other important molecules have ring structures. Planar means that the ring is flat and not bent. (see Figure A)
| Firgure A: (ii) a 2D representation of the cyclohexane in part (i). Even though it can be draw to look like a planar molecule in 2D, the actual 3D shape of cyclohexane is not planar. |
If you recall from high-school chemistry, atoms have electrons arranged pairs that exist in orbitals, that fill from the lowest-state energy to the highest state energy. The first kind of orbital is the s-orbital, which is basically spherical (Figure B). Each electron shell has only one s-orbital.
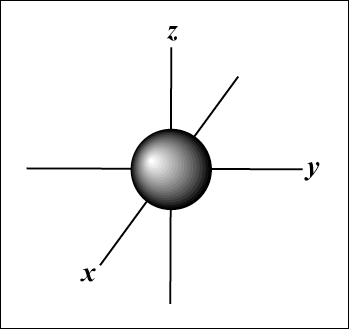 |
| Figure B: the s-orbital |
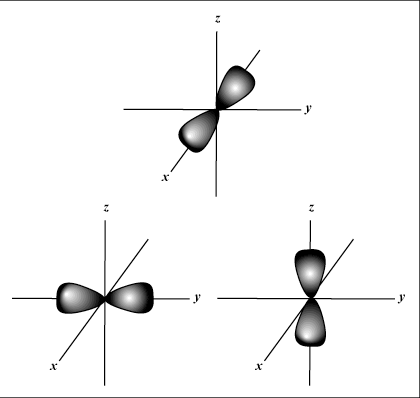 |
| Figure C: the three p-orbitals lie along the three axis of spatial dimension. |
 |
| Figure D: (left) The top view of a six-carbon ring showing the connectivity between the carbon and hydrogen atoms. (right) Side view of the ring with the p-orbitals present. |
 |
| Figure E: the blue stick and sphere structure is the six-carbon ring; the red spheres are hydrogens, and the aqua-green sections are the two p-orbital cloud rings. |
When this p-orbital ring is uninterrupted by missing orbitals, and there are an odd number of double-bonds, the electrons in the double bond are no longer restricted to the bond between two atoms; they become free to move in the p-orbital ring and thus end up running in circles around the ring very very quickly, which adds a BOATLOAD of stability to the ring and makes it very unreactive.
Aromatic rings are usually mostly made of carbon, but can also contain nitrogen, sulphur, and other non-metallic elements. Examples of important aromatic structures include DNA nucleotides.
 |
| All of the rings that you see here in the nucleotides that make up DNA are in fact aromatic rings |
PS - Sorry for the poor photo qualities for some of them; I don't know how to fix it, but if you click on the photos they will enlarge onto a white background that makes it easier to see. Sorry about that!
~~~~~~~~~~
Imaginism sounds cool! I like the poem that you posted, and thank you for the short explaination afterwards; I was confused because I didn't understand what the imagery was comparing in the poem until you said that the imagery describes her feelings and emotions. It is very cool. I wish I had the time to be able to study things like this.
SO I'VE ALMOST SURVIVED MIDTERM SEASON =D
As you know, on friday I had a midterm on Fungi. It was challenging and quite long; I barely had enough time to finish all of the questions, and I wasn't sure if I answered the written questions with enough detail or not, which made me nervous. When we got it back yesterday, the prof said the midterm average was 57% =S I was so nervous that I didn't look at my grade until I got home and forced my parents to look at it first (92%, which made me so happy that I almost died from relief)
Tuesday was the organic chemistry midterm. That was also quite challenging; the midterm covered two topics: organic acidity/alkalinity and organic reaction mechanisms. I expected the midterm to focus more on mechanisms (because all of he practice midterms focused on that) but there was more emphasis on acidity/alkalinity, so I didn't go as well as I hoped, but hopefully I didn't mess up too much.
EDIT: I GOT MY CHEM MIDTERM BACK TODAY. 83% =D
Yesterday I had my Multivariable Calculus midterm, which I was freaking out over because I suck at multivariable calculus, but it was easier than I expected it to be, and thus I really really really hope that I did well on it too.
This morning I had my fungus lab exam. Slightly horrendous. I was prepared, but not as prepared as I should have been; there was one organism that I could not identify for the life of me, even though I remember looking at it in lab a few weeks ago and answering questions on it. It frustrates me that this lab exam is worth 20% whereas the written midterm I wrote last friday (the one I got 92% on) is only worth ~10% =(
I now only have two midterms next week to worry about: Cell Biology and French Contemporary Language and Literature. I'm totally going to get raped by the cell bio one because I've been neglecting to do any of the readings and extra work (I really dislike my professor and thus I am not motivated to learn) but the french midterm only covers the present and past tenses, grammar only (no comprehension or essay writing) so I'm not so worried about that. I'm doing surprisingly well in French, considering I haven't taken it since gr 12, so I'm quite happy =)
Anyways, I'm going to let myself relax until the weekend, where I'm going to be kicking it into high gear again to prepare for next week's midterms.
I hope everything at Trinity is going well for you; I saw the photos you were tagged in from thanksgiving weekend and it looked like you had a great time!
Chat soon,
~Tim~
PS - I just discovered yesterday that "a beached whale" translates into french as "une baleine échouée" which literally means "a failed whale" (baleine = whale, échouée = conjugation of the verb meaning "to fail")

No comments:
Post a Comment